|
FAQs on Sulfur-Based Denitrators,
Denitrification
Related FAQs: Denitrification
Gear, NNR (Natural
Nitrate Reduction, Anaerobic Bacteria), Biological
Filtration, Deep Sand Beds,
Fluidized Beds, Ammonia, Nitrites, Nitrates, Phosphates, Wet-Dry
Filters, Bio-Balls,
Related Articles: BioFiltration,
Nutrient
Control and Export,
|

|
Sulfur denitrification
12/5/19
Dear Bob,
<Hey Branko>
We are thinking of adding Sulfur reactor to battle nitrates in our fish
system.
<Mmm; can be done... though Nitrate influence is not often much of an issue
with captive fishes>
Do you have any insight, how would copper that we dose react if sulfur
reactor is introduced. Would sulfur bind copper to it?
<Mmm; yes... making sulfur oxides... >
And if yes would it eventually become full like filter media so we can dose
needed dose after media gets filled?
<How to put this... how much of a problem, negative influence, what I would
do is experiment a bit if you're determined to use a sulfur denitrator... to
determine how much, how often you'll need to renew/adjust the (chelated)
copper to keep a physiological dose present. Bob Fenner>
Re: Icp test questions... S2 issue
2/19/19
What are some sources for boron and ways to remove it?
<I would stick to frequent partial water changes, use of PolyFilter,
Chemi-Pure or such IN a biological system. B>
Re: Icp test questions 2/19/19
Now would lent ph buffer be my source?
Was just reading an arrival from randy Holmes on effects of boron levels
<... What? "Lent"?... Do you mean Kent?
http://www.integrachem.com/msds/B8579Q_26698_101.pdf
Do you see Sulfur listed as an ingredient? I don't.
B>
Re: Icp test questions 2/19/19
> Subject: Re: Icp test questions
> Now would lent ph buffer be my source?
> Was just reading an arrival from randy Holmes on effects of boron
levels
> <... What? "Lent"?... Do you mean Kent?
Yes Kent dhk buffer also looks like calcium reactors can be a source
according To the article if it is not coming from the source water
>
http://www.integrachem.com/msds/B8579Q_26698_101.pdf
> B>
<Please write out your queries. >
Xport NO3 Product; Sulfur
7/1/17
Hi,
I was in my LFS yesterday and was told about Brightwell aquatics Xport
NO3 product which comes in a few shapes and sizes. It is a porous media
containing sulphur which sits in a low flow area of a sump and
denitrifies they tell me.
<Well-stated; and yes to there being such sulfur products. Best used in
reactors... where they receive more circulation>
I can see no references to it in the wetweb pages and though there are
threads on it elsewhere the question of does it actual make a
significant impact on NO3 remains largely unanswered. I do not want to
waste my money as this hobby is full of 'miracle' products.
<I think what little we/WWM have is likely archived here:
http://www.wetwebmedia.com/SulfurDenitratrF.htm
I have a 180 gal FOWLR which despite water changes always has high NO3.
I want to eventually keep some soft corals which requires me to sort out
my water parameters.
<There are a few approaches. Am a bigger fan of natural methods... deep
DSBs of fine material, RDP refugiums with macro-algal culture....>
I have tried vodka dosing and biopellets in the past, Cyano loved them,
no other change. I have a good skimmer too, Bubble Magus curve 9. I have
reduced my tank PO4 to 0.5 ppm with ferric oxide which greatly reduced
the
nuisance algae but though the PO4 is not rising the algae seems to have
found a new lease of life. I have read this can be due to PO4 leaching
out of my live rock but I'm not seeing PO4 increases when testing?
<Likely being "scarfed"/scavenged readily by the algae/Cyanobacteria...
a dynamic process>
With denitrifying media low flow is desirable but no flow is pointless.
<Ahh; again; well-stated>
As sump space is limited I was wondering if putting such media in the
bottom of the overflow chamber is an option?
<Yes; tis one... though do read the citation above, consider a chamber
with directed flow>
I have no idea what the water turnover is there though as the weir pipe
draws from the surface of the overflow chamber about 1.5 ft higher up.
Thanks for your time and help,
Toby
|
Nitrates gone wild! 3/6/17
Hello, and thank you for what you do.
<Hi; welcome Karen>
You've been a great resource to me over the years - but shamefully, not in
the last few. which is why I am
writing today. I will try to give a bit of history without writing a novel.
I have a 225 gallon tank with probably a 30 gallon refugium and an
additional 40 gallon sump. I say probably, because I upgraded from
a 90 gallon in 2009 and I truly don't remember all the details. Tank has
approximately 300 - 400 lbs of live rock and maybe 200 lbs of sand (all was
purchased from a company called Tampa Bay Saltwater). There's between 2 - 3
inches of Miracle Mud in the refugium along with some of the above mentioned
live rock. I was very involved in the hobby until probably 2013 or so when a
number of things happened (including the earlier death of my husband) to
draw my attention away.
<Reasonable>
At some point, all of my corals died and I was left with a fish only tank -
some of the fish having been in the earlier tank.
We (the fish and I) kind of limped along until last September 2016 when my
lighting system failed. I had one of the first LED systems - you may
remember the Solaris? - and it finally bit the dust. At that point I had
these 8 and 9 year old fish (Regal Tang, Saltwater Tang, GSM clown, 2
Ocellaris clowns, One Spot Foxface, and a foot long Engineer Goby) and I had
to make the decision as to whether to rehome them and dismantle everything
or sink some money into the tank. I chose the latter and replaced the
lighting system and had my local LFS who has helped me throughout the years,
come and do a thorough cleaning and reboot (so to speak) of the system.
The LFS is also using my tank for some of their larger soft corals in hopes
of later fragging them - or maybe they're just being nice because I've been
with them forever.
<Neat>
Of course, within two weeks of replacing the lights, both my skimmer pump
and main pump also died and had to be replaced.
After all that happened, and after several water changes within a couple of
months, my Regal Tang got a mild case of ich. Acting on the advice of the
LFS, I treated the tank with Kick Ich
<Ughh! A placebo at best. See WWM re this sham/scam>
and upped the feeding, varied the diet, added Selcon and VitaChem to keep
the tang's immune system up.
<These will help>
I realize that's not the treatment of choice but I've been out of the hobby
for a long time and, well, insert whatever excuse you can think of. At the
time, the water was tested and everything was within normal limits but the
nitrates were very high (around 100) but that was attributed to all the
stirring up and the feeding.
<Likely so>
Added Chaeto to the refugium and a carbon/GFO reactor and UV sterilizer.
Ultimately the tang cleared up and presumably all was well for the next 3
months. I added a little ORA Orchid Dottyback and she's doing great - except
that she was so small I continued to overfeed to ensure that she was getting
food. Please note that during all this there wasn't any further water
testing going on, but all the fish (even the Regal eventually) were fat and
happy, the mostly soft corals thriving, and the water looked great. I'm
running a skimmer, the UV sterilizer, and the carbon/GFO reactor. I have a
moderate CUC consisting of a couple serpent stars, 50 -100 various snails,
and a few Peppermint and Skunk Cleaner shrimp. So that's the history, and
here's the problem.
I purchased a Mandarin and placed him in the tank, thinking that an 8 year
old tank with lots of live rock, etc. would surely support him. He's a big
guy, approximately 3+ inches. But I started thinking that he was looking
thin. So I bought some pods and put some Chaeto in the refugium. Then I
bought some more pods. Then I bought an additional 50k pods and some
phytoplankton and he still looks thin to my mind.
<I'd place the Mandarin in your refugium for now; likely interstitial fauna
there... AND I want to make sure and mention that I'd increase the depth of
the DSB there, AND run the lights/lighting there alternating with the
main/displays (RDP)>
So I had an epiphany (I know, I know) and tested the water and the nitrates
were very high (100 ppm). and I'm now freaking out because there's no way to
know how long they've been that high - could have been for a long time or
since I started paying more attention to the tank and began way overfeeding
to bolster the tang. I also don't know if the Kick Ich treatment killed a
lot of the beneficial bacteria or the existing pods or ..
<Perhaps... but... I would NOT obsess re the high NO3. It by and of
itself is not a major concern; how to put this: OFTEN what such readings
"co-interpret" is an abundance of other ills; high dissolved organics
period, low DO, high BOD, low RedOx...>
Thus far I've cut back on feeding, am upping my water changes (but am
somewhat at the mercy of the LFS who does my water changes)
<Mmm; I'd take all this back. Do the maintenance myself>
and I started the Prodibio Bioclean S on Friday. I've also added more flow
and I took some of the live rock out of the display and put it in the
refugium. And now the Regal Tang has started flashing on the glass and
acting
differently - but only when the lights are on. Appetite is still good and
there are no spots, but she's banging up her head a bit. I don't know if
that's oncoming disease or just a stress reaction to the change or both.
<Can't say>
The guy with the LFS that's doing the water changes is concerned about the
tank because the rock is sitting on egg crate and he believes that that is
contributing to the nitrate issue by allowing detritus to accumulate,
especially under the rock.
<Possibly.... I might well remove it in sections; even half during one
maintenance interval, the other next>
So now I'm at a place where I don't know if I should dismantle the tank and
try to remove the egg crate - and likely stress my elderly fish beyond the
limit; add more sand on top of the existing sand; add a sulfur denitrator,
or ..?
<Or all three...>
I know I need not take a scattergun approach and randomly try a bunch of
different things (I already feel like I'm starting to go down that rabbit
hole). Parameters today (water change about 48 hours ago) are: Ammonia and
nitrites 0, pH 8.2, Alkalinity 11.2, Salinity 1.25, Calcium 480, Magnesium
1280, Phosphate .25 and Nitrates 160. Attaching a few pictures of the tank
so you can see the amount of rock, sand, etc.
<Actually; looks fine macroscopically>
Any guidance you can provide would be very much appreciated. I'm sorry for
the long email, but I was trying to cram in 8 years. And again, thanks so
much for all the valuable help you provide.
Regards,
Karen
<Thank you for writing, sharing. I would proceed as mentioned and hinted at.
PLEASE do communicate if this message is not clear, complete and/or you have
other concerns, developments. Bob Fenner>
|
.jpg) |
|
Re: Nitrates gone wild! 3/7/17
Thank you so much for your quick reply, and thank you for easing my panic.
I've read through this several times and I'm trying to come up with a plan -
additionally, as I'm sure you saw in the pictures - I have a Sailfin Tang,
not a Saltwater Tang �� I also have a few further questions, if I may ask?
<Go ahead>
I'm thinking that prior to moving the Mandarin to the refugium, I should
increase the depth of the DSB as advised, and since it's completely made up
of Miracle Mud, is that what I should add rather than sand?
<I would CAREFULLY (with the sand washed, damp... in a plastic hand-sized
container) lay the new sand atop the mud. The latter will dissolve,
decompose for the most part in time; and do some good here>
I mentioned three possibilities (removing egg crate, adding sand, and adding
a sulfur denitrator) and I interpreted your response to mean that it would
be a good idea to do all three?
<Yes; any or all>
Removing the egg crate can be done although it will be a major undertaking,
particularly since some of the rocks are huge (probably 30 -40 lbs). I'm
worried about the stress on the tank and the stress on the fish in
proceeding in that direction... but if it needs to be done, so be it.
<Doesn't "need" to be; just would be better. The other measures mentioned
above/prior would do ninety some percent of what can/could be done to
improve water quality here easily>
My preference would be to add more sand without removing the egg crate,
<Then that is what I'd do>
but I don't want to waste the money or the effort in adding new sand if I'm
only covering up the problem.
<Understood>
The sand in the tank is not quite a crushed coral but looks a bit like it,
only a tad finer ... as described by the website where I purchased, it's
..." harvested well offshore in the ocean to be clean of pollutants and
silicates. Live sand collected in this manner will be a mixture of sand,
shell bits, corallines, bivalves, starfish, snails, and many organisms not
visible to your eye". Would you suggest ordering more of the same or using
another sand?
<More of this, finer in grade if available... most under 1 mm diameter>
And the third option is the sulfur denitrator. I've been reading up on them
- but is there a specific model that you would recommend?
<Mmm; hold off on this till the other work is done and a month or two has
gone by. Again; this is what I'd do>
Is there anything I have missed?
<Not that I perceive>
Again, I'd really like to thank you for your assistance with the tank. I get
ridiculously attached to these little creatures and I really do want to
provide an appropriate environment for them, despite appearances to the
contrary as evidenced by my neglect the last few years.
<A pleasure, indeed honor to share with you>
Despite that, they've hung in there with me ... the Foxface is actually the
first saltwater fish I purchased well over 10 years ago.
We all thank you.
Karen
<And all are welcome. BobF>
|
Sulfur denitrator... input diff. than WWM; but what is it?
1/26/15
Hallo,
<Ave!>
Belgian Anthias is the name I use on several forums and I
propagate the use of BADESS ( Biological Autothrobe Denitration by
Elemental Sulphur) to solve the nitrate problem for ever.
<There's a Beatles song refrain in there as well as a few pub jokes I
suspect. Oh! And am making moules and frites for a run ending tomorrow
eve at Green Flash Hash>
Reading FAQ's concerning sulfur de-nitration and the answers given on
these questions on WetWebMedia let me decide to ask this question: has
Bob Fenner or one of his panel ever used BADESS to lower high nitrate
levels or used BADESS to keep the nitrate level very low removing high
daily nitrate production?
<I have not personally; have only chatted w/ others, read re>
From what I read I may conclude that this is not the case because a
sulfur denitrator will not work and can not be managed satisfactory the
way it is advised on your website. BADESS is very easy to manage and
very reliable but only when enough sulphur is used and the reactors are
big enough for the purpose they are used for. Carbon dosing makes it
very unreliable and very difficult to manage.
<I do agree w/ the last>
A sulfur-denitrator must not be mixed up with a carbon de-nitrator and
both reactors must be managed in a different way. BADESS makes it
possible to have full control over the nitrogen cycle in an aquarium
system on a very easy and practical way but not when the advise given on
your website is followed.
<Do send along your corrections and we'll add>
Sincerely Yours
Belgian Anthias
De Mille CMF
<Bob Fenner> Sulphur Denitrator
6/17/14
Hi Guys
<Hey Arun>
First of all let be congratulate you on proving a stellar website for
novices as myself.
<Ahh>
I have a relatively simple question, having recently purchased a used
Korallin denitrator I set it up with the existing media having run it
for about 6 weeks I still cannot detect a reduction in the main
displays nitrate level.
<Mmm... Perhaps the media... might I ask you to measure the
nitrate and pH of the water just exiting the reactor?>
I have decided to change the sulphur media ,
<Good>
not knowing how long it has been used for, when I researched the product
I came across sulphur prills used primarily by horticulturists ,it
states that it is 99.5%pure sulphur, it is a fraction of the price of
the packaged
products sold by the online aquarium stores.
<Ah yes>
Will this product be safe to use, and based on what I have read can you
advise me whether the packaged aquarium sold sulphur is coated with the
nitrate eating bacteria?
<It should be safe to use, though I might test a batch on a small
experimental set-up (not on your main/display)... "just in case". And no
to bacteria being applied to aquarium sulfur products as far as I'm
aware/have seen>
Thank you Arun Sharma
<Welcome. Bob Fenner>
Re: Sulphur Denitrator
6/17/14
Thank you Bob
<Welcome Arun>
Sulphur denitrifier, use with sulphur, or bio pellets?
11/17/13
Hi Bob,
?????????? I am hoping you can help me once again. I have a 180 gal
FOWLR that's been running for about 18 months. It always has high NO3,
fish are well fed and healthy but I would like more control of NO3 than
reduced feeding and big water changes would give me. I have tried carbon
dosing for several months now without change
<Doesn't always "work"... only in/w/ systems that are carbon deficient>
in my parameters using Red sea's Nopox liquid, I read people have
success
with carbon dosing sooner than this, but I have not been so fortunate. I
have two skimmers running in my sump, Red sea's C-Skim 1800, and an old
TurboFlotor 1000 which uses ozone, plus? Plenty of live rock so it should
work. The skimmers certainly pull out plenty of skimmate.
?????????? I have an old Korallin sulphur denitrator from an old tank
currently in? Storage.
<I'd get it out; try using it here>
It was fiddly to operate and sometimes smelly, but good at reducing NO3,
though I was always worried about the acidic effluent affecting
alkalinity.
I am debating? If I should resurrect this denitrator? For my current tank.
The denitrator? Recirculates tank water within itself via a pump, but?
has
a low flow rate in and out for the anaerobic bacteria it utilizes. I was
wondering if I could use bio pellets with this denitrifying filter
instead of sulphur.
Would that work?
Are bio pellets preferable to sulphur denitrification??
<... depends on the make-up of the system, cause of issues... the two
mechanisms mentioned are not dependent on each other... Operate
independently. Sort of like asking re using a bike to get about vs.
using a blender to make a drink>
Would be interested and thankful for your advice,
?
regards,
?
Toby
?
<?Bob Fenner>
Sulfur Reactor 6/28/13
Hi Crew!
I have a sulfur based nitrate reactor (Korallin brand) that I would like
to use on my saltwater reef tank. It is extremely difficult to find
useful (and well cited) articles on this subject. I read all of the
FAQ's about this topic on your page, but cannot seem to find any
articles about it. I do have a few questions about the process and would
like better clarification. First, does the unit release a toxic
sulfur compound back into the aquarium? I have read some claims that it
does, and other claims that it does not.
<It does not... unless there is some gross mis-use of wrong media...>
Second, is the reactor safe to use even after the nitrates fall
to an appropriate level, or should the process be discontinued until
when/if there is another nitrate spike?
<It is safe to use continuously>
Next, the ph leaving the reactor was 6.0-ish, and that is
obviously very low, but I cannot seem to correct the imbalance. The
instructions mentioned to have 2/3 sulfur and 1/3 calcium carbonate,
<What is the source... the brand, make-up of the CaCO3? Some types are
varyingly in/soluble. You may need to have a higher ratio of
carbonate... Otherwise, I'd consider running the water through a bit
more quickly (let's say so the pH is 6.5 or so) and baffling/aerating
the water at the discharge point to further elevate pH>
but in an attempt to correct the low ph produced by the sulfur, I did a
50/50 mix, and the ph is still low (roughly 7.0).
<This should be fine>
So should I use even less sulfur and more calcium carbonate, or is that
defeating the whole purpose of the unit to begin with?
<Not defeating; other than diluting the Sulfur exposure per pass>
Finally, can the calcium or alkalinity be negatively affected by this
process, or will they remain more stable because of it, or is it not
going to really impact the system at all?
<Both alkaline earth (Ca, Mg...) and Alkalinity can be (dangerously)
reduced (via acidification)... You should be measuring all to assure
these are not dipping too much, too fast>
I appreciate your time, and help with my questions, and hopefully this
will help other people just trying to get the basics of sulfur
denitrification and its effects on the tank parameters other than
nitrates.
Katy
<Welcome. Bob Fenner>
|
DIY Sulfur reactor design - request schematic review?
3/2/13
Bob, at your prior suggestion to me on nitrate reduction, I now have a
DIY biopellet reactor running. Seeing that I could implement a sulfur
reactor using similar (cheap) materials, I have all on order and wonder
if I could trouble you for a quick design review and a question
<Sure; have looked over>
I have. Also, to point out, each will be undersized (slightly) for my
system, which may serve to insulate me from overly stripping nutrients
from my water (?).
<Might well help>
I can't expect that to be a problem in my system of messy eaters where
nitrates still hover around 20-30 after large (30-40%) water changes,
but time and experimentation on throughput will tell...
<Yes>
Anyway, on to my design. See the attached, which hopefully is self
explanatory. For my 200G system, I plan to use 1.2L sulfur and 1.8L of
course aragonite media. My design deviates from common sulfur reactor
practice in that there is no aragonite in the sulfur chamber (i.e.,
which recirculates). Effluent from this chamber drips to an aragonite
chamber (fed at bottom), where it will gravity drip (out of top) to the
system.
<... gravity feed? The system water will be pumped through the S2
reactor and then through the aragonite, yes?>
Now to my question. Is this amiss for not having the aragonite in the
recirculation chamber?
<It is not. In fact, your design is better... for isolating the two
media>
Should I add some aragonite here, or reduce sulfur to a level where I can
have a ratio of sulfur/aragonite subject to the same chamber
circulation?
<I would stick w/ what you show for now>
Any other design concerns?
<Just the drive of sulfur into your system... go slow flow wise, measure
the effluent pH...>
Thank you!
<Welcome. Bob Fenner>
|
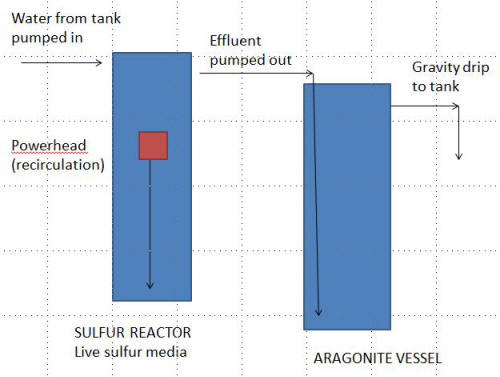 |
Re: DIY Sulfur reactor design - request schematic review?
3/2/13
Bob - thanks! To answer -
<<Welcome>>
<... gravity feed? The system water will be pumped through the S2 reactor
and then through the aragonite, yes?>
The system water will be pumped through the S2 reactor and to the aragonite
vessel (entering at bottom). The aragonite will overflow and drip into the
tank (i.e., no pump within it).
<<Ah yes...>>
Should I go with this plan?
<<Looks fine. B>> Re: DIY Sulfur
reactor design - request schematic review? 7/12/13
Bob, hope all is well. Question on sulfur reactors and use of GAC within...
<Ok>
Is this a concern? On occasion, I have had hydrogen sulfide odors coming
from my sulfur reactor. This has primarily been a result of flow tweaks, but
also I have had problems with the tank water supply pump clogging (and/or
inadvertent pump power shutoff).
<Mmm, well; if only "a bit" (humans have quite sensitive/acute sense of
this, other S containing cpd.s), not a big deal... IF filling the house w/
the odor, an issue>
I have noticed that temporarily placing a bag of GAC within the chamber
helps me get the reactor back to normal.
<Yes>
Is there any concern with just running the sulfur chamber full-time with
GAC present?
<There is not (as far as I'm aware of course). Have seen hobbyist to large
commercial reactors w/ a chamber of GAC as a finish contactor many
places/times>
Thanks.
<Welcome. Bob Fenner>
|
Adding co2 to a sulfur denitrator? --
10/21/2009
Dear WWM
<Larry>
I have a sulfur denitrator which uses a Korallin 1502 shell. I was
wondering if the denitrifying bacteria that live in the sulfur can live
and work in a co2 enriched environment?
<Yes... often carbon is a/the rate-limiting (Amtal Rule) source
material; hence folks feeding sugars, alcohols...>
The reason for injecting co2 would be to increase the calcium output of
the denitrator.
<? Do you have a source of this alkaline earth element in your
reactor?>
My well water even after my R.O. and polisher has a ph of 7.8 with the
denitrator I can push it to 8.0 with out buffering. After that it
requires buffer which drives up my ALK. The system is 240 gal with a
150 gal. refuge.
Lots of circulation in main tank. I have about 68 mixed corals, 8 fish
largest about 3" in addition to a fair clean up crew. I can do
this it would save adding a separate calcium reactor. Thank you.
Larry
<Mmm, if I'm following you here, you can likely improve your
system overall with some careful (metered) input of carbon dioxide
being fed into the reactor... I would mix some sort of CaCO3 in with
the Sulphur beads. Bob Fenner>
Re: Adding co2 to a sulfur denitrator?
10/21/09
Thank you Bob
<Welcome Larry>
The sulfur denitrator I have is a new innovation designed and tested at
the aquarium of France. It works exactly like the Korallin 1502 calcium
reactor.
The difference is the bottom 1/3 is sulfur beads the top 2/3 a calcium
media like ARM in addition there is no Co2 added.
<Ah yes, have seen this... the CO2 can be scavenged/released from
the calcium carbonate/ARM... in the reductive ("acidic")
circumstances in the contactor>
. There are no sugars added like many sulfur systems. I hope this
clears up any confusion about what I have.
Thank you again for your help.
Larry
<These units can/do work under some circumstances. BobF>
Re: Adding co2 to a sulfur denitrator?
10/21/09
Thanks Bob
<Welcome Lar>
If I understand you right you don't feel that the addition of Co2
gas will hurt the bacteria in the sulfur bed.
<Up to a point, it should help... You don't want to add so much
that the pH drops precipitously...>
I would set the Co2 gas injection system up just like a normal calcium
reactor. Using a bubbler and a ph
monitoring system like the 122 with slave valve for the gas. I would
start with 5- 10 bubbles/min.
<This should be fine>
I just wonder why no one has done this before!!
<I do think it has... Not likely commercially viable commercially,
as the CO2 cylinder purchase/lease, regulator, needle valve, monitor...
would put the cost beyond what most folks would pay. BobF>
Larry
Re: Adding co2 to a sulfur denitrator?
10/21/09
Bob
I have used this sulfur denitrator in my old 75 gal tank with a 30gal
refuge.
It worked great and kept my nitrates at 0 and calcium a little high if
I didn't watch it. The 75 was wall to wall corals and about 25
fish. I swear by them. It just won't provide enough calcium for my
combined 400gal
system.
<Perhaps a larger unit... or "ganging" another all CaC03
unit after the denitrator... or a better, more soluble source of
carbonate (try Korallith, Knop's product...)>
I can either buy a calcium reactor or if I can get a little more out of
the sulfur denitrator by adding Co2 I won't need to.
Thank you
Larry
<There are other approaches... do consider the few I've
mentioned here. BobF>
Re: Adding co2 to a sulfur denitrator? --
10/22/09
Bob
<Big L>
Thanks for the info. I just purchased a Korallin 1002 I will add it to
system. Then maybe I will experiment with the Co2 in the sulfur system.
It would be an interesting concept if it works.
Larry
<I do agree... and would like to relate that like A. Einstein et
al.s "radical" designs for refrigeration (yes, including
nuclear)... I've seen some very adventurous denitrator
engineering... A few Interzoo's (Int'l industry/trade show)
back, I saw a hand-blown glass menagerie from an Italian co. that fed
H2SO4 (yep, Sulfuric Acid) into a carbonate source to feed such a
unit... Wowzah! B>
Cool!
Re: Adding co2 to a sulfur denitrator? 10/24/09
Bob
<Hi there Lar!>
It has been a pleasure talking with you. It is nice to meet someone in
this field who is not entrenched in the tried and true and keeps an
open mind to new innovations and ideas.
Thanks
Larry
<I will state the same re my impression of you, yours. Cheers!
BobF>
New-Fangled Denitrator Thingy...? Hi Folks: <Hi there!
Scott F. at the keyboard today!> Always new gadgets showing up on
the market. This time its a Sulfur Denitrator. What's your take on
this item, anything to it? Pro, cons, and etc. As always I appreciate
you comments. Rick Luckert <Well Rick, I am not familiar with the
particular denitrator that you're referring to here. However, I
have seen a number of "denitrator" products on the market
over the years, most of them reasonably effective, all of them seeming
to require considerable maintenance and "tweaking" to do
their jobs effectively! Frankly, I believe that a well-maintained deep
sand bed, either in the display or in a remote location (a sump or
refugium), is the best approach to denitrification. When coupled with
regular maintenance techniques (water changes, protein skimmer
cleaning, etc.), you'd be hard-pressed to find a better system to
do the job, IMO! That's my two cents on the subject! Regards, Scott
F.>
Sulfur denitrification Hello, Sorry for the msg.?
and thank you for your time. <No worries and glad to
help> I was reading and I found material about the sulfur
denitrification (using sulfur as media for bacterial colonies). I know
that the best against pollution is dilution and the control of
nutrients, but in my country (Argentine) is very expensive the RO water
and equipment, and for that reason I'm always looking for new
method of improvement, and also I am a DIY guy. For that reason tell me
what do you think about the sulfur denitrification.. <Quite
popular in some countries in Europe... but virtually unknown in the
U.S.> MY system is 50 gal FOWLR with a few snails and a Condy
(with 100W halogen lamp over), all over Crushed shell, no coral sand
and DSB, because is too expensive. Do you think that is better
remove the crushed shells and leave the bare bottom with the LR in
order to reduce the nitrate level(50mg/l). <Mmm, that or make
the bed pretty deep... several inches if the average particle size is
more than 5-6 mm.> I do siphon 5 gal every 2 weeks but is
always the same , the level goes up. Thank you in advance and
sorry for my English. Eckhard <No problemo. Yo entiendo
todo aqui. What are your actual nitrate readings? Have you considered
making a denitrifying bed in a sump/refugium and tying its volume to
your main system? Bob Fenner>
- Sulphur Reactor De-nitrification - Hi Guys. I am relatively
new to reef keeping (11 months) and have just invested in an Aqua Medic
Sulphur reactor in an attempt to reduce my rising nitrate levels
(currently 50ppm). I have recently set the unit up at 1 drop per second
flow rate while it matures. I have, however, noted that the
instructions state that you should also pump the water that leaves the
filter through a bed of hydrogen carbonate to neutralize the by-product
of the process, sulphuric acid. <Sounds reasonable.> I assume
that this has been suggested to avoid a reduction in PH levels?.
<Among other things - alkaline reserve.> I have checked the
instructions for other units and have not identified any similar units
which state this as a particular requirement, however, they do state
that PH levels of the water leaving the unit will be reduced. I would
be grateful if anyone could advise on whether this is a requirement
specific to the AquaMedic unit or whether all units require some form
of secondary filtering to increase PH. <Given the nature of the
byproduct of this type of reactor, I'd think some type of secondary
reaction to reduce the strength of the sulphuric acid would be most
useful.> My initial view was that I would compensate any effect on
PH by buffering the water as well as ensuring that the return tube from
the filter feeds into a high flow area of the tank. <For certain,
you're going to have to do something... your pH will hit the floor
eventually if you don't.> In addition, does anyone have any idea
what the maximum flow rate through the unit is once the reactor has
started to work. <No... should check with AquaMedic on that one.>
I assume that you are not limited to 1 drop per second as maximum flow?
<Probably not.> Any help would be much appreciated Thanks Jason
<Cheers, J -- >
- Sulphur Reactor De-nitrification, Follow-up - Thanks for
the quick response, <My pleasure.> looks like in going to have to
go for the additional unit. Better to be safe then sorry.
<Agreed.> Cheers Guys <Cheers, J -- >
Help with DIY of sulfur denitrification Hi, <Hello
there> I'm a new visitor to your site and find it very helpful.
<Ah, good> I am designing a sulfur denitrification system. I
bought 3 six-foot clear plastic tubes that are six inches in diameter
each. It has been suggested that start the water flowing through one
tube filled with sulfur beads. Then I am to send the water through a
second tube filled with calcium carbonate sand. This should return the
water to normal pH while dissolving part of the sand thereby, raising
calcium levels. Any comments? (Please try to limit your laughter.)
<Mmm, not a laughing matter, particularly should you not remove all
the sulfur by-product...> I need help to calculate the approximate
water flow rate. I can calculate how long it would take water to pass
through the first volume but do you know how long it will take to turn
anaerobic? <Too many variables to consider... I would experiment
here... with measured flow rate/s, a time device with a second
measure... and either dissolved oxygen and/or pH as a measure of
anaerobiosis> Do you have any suggestions for using part of the
third tube to create a fluidized chamber? Could I use excess CO2 that
comes from the sulfur bed? <I would have an "ammonia
tower" arrangement... a reverse flow oxygenating device to blow
air up as the water is cascading down plastic media...> Thanks a
lot, Asa <Please keep good notes, records of your activity, thoughts
here, and send along your results if you will. Bob Fenner>
Denitrators. Does anyone know anything about
denitrators? - 07/22/07 Hi, Does anyone know anything about
denitrators? <Quite popular in Europe, especially in ancient times
when live rock wasn't widely available. See
http://www.wetwebmedia.com/denitrification_erfaqs.htm for answers to
the most common questions.> and do you know which denitrator is the
best on the market? <It certainly depends on the size and type of
system. For small and medium sized reef and FOWLR systems up to 250
gallons (and possibly even larger) I'd prefer live rock, DSB and a
large refugium (possibly with some macro algae), this combination is
better and more cost efficient. A good skimmer will also be very
helpful with any nitrate issue.> I have been looking at one made by
Reef Octopus and it uses Sulphur. <Do they still build them? See
their homepage. I do not have experience with this specific product,
but the newer ones are very similar (disclaimer: manufacturers may
disagree). A problem I see with some models is that the RedOx potential
(see http://www.wetwebmedia.com/redox.htm if unknown to you) should be
monitored and there should be some auto-regulation to avoid toxic water
(nitrite, ammonia) getting into the tank. Usually simply some type of
aeration is applied at the water outlet to ensure everything non
gaseous is turned to nitrates again. What if for some reason the
aeration fails or drops and there is no monitoring? As stated above,
I'd go with other types of natural nitrate reduction for most home
systems and leave denitrators with Sulphur to very large high end
systems where live rock cannot be used in serious amounts.> Any help
would be appreciated. Thanks, Maison. <Hope that helps.
Marco.>
|
Sulphur bead nitrate reactor producing instead
of reducing nitrate - 08/02/07 Hi folks, <Hi Rob.> I
finally got around to building the ozone/nitrate reactor I
emailed Bob about on http://www.wetwebmedia.com/redoxinst.htm.
The beast has been built more or less to specification
(photograph attached). In the bottom left you can see the nitrate
test of the effluent. Oh dear. My tank previously tested at
around 5 to 10ppm. After four days of operation it's now
about 25ppm and climbing. The nitrate level of the effluent is
immeasurably (at least by my Hagen test kit) high at well over
110ppm. The Cyanobacteria is growing incredibly quickly. I
haven't yet fired up the ozone generators so really this is
just 2 litres of pelletised, activated carbon, 7 litres of
Sulphur and 7 litres of coral chips in series. The drip rate is
around 5ml per second which is a bit high but still tolerable.
This services around 500 gallons of moderately stocked and fed
reef. So where's the nitrate coming from? I've tested
each chamber and the nitrate appears only after the Sulphur. The
Sulphur beads are home made but from quite pure (BP grade)
Sulphur. <I'd mix some of the Sulphur with freshly mixed
saltwater, move and aerate it for at least 24 hours and test for
nitrates. I'd also test the carbon.> It's
"making" nitrate from the water that passes through it.
I've read the nitrate can spike during the bedding-in period
of a Sulphur denitrator <Yes> but this kind of rise seems
extreme. The Sulphur chamber effluent has significantly raised
levels of both ammonia and nitrite and the pH has dropped half a
point so presumably bacteria are metabolising dissolved organics
passing through it. <Likely and possibly also material from
carbon or Sulphur.> Why in the Sulphur chamber, though? The
activated carbon has a much larger surface area for aerobic
bacteria colonisation. Why aren't I seeing a spike after
that? Is Sulphur a significant enough bacterial accelerant to
cause this kind of effect? Can I expect a general consumption of
dissolved organics instead of a discrete, mostly nitrate
metabolising function using Sulphur? Many thanks in advance, Rob.
<It's all a question RedOx potential as you know. To be
absolutely sure what is going on, you will have to measure it. A
denitrator of any type can only reduce nitrates effectively
between -100 mV and -200 mV turning nitrates ultimately to
gaseous nitrogen. Anything above -50 mV will reverse the process
to ammonia->nitrite->nitrate just as in a standard filter
and consequently produce nitrates (flow needs to be decreased,
likely what you are observing right now). Anything below -300 mV
will provoke bacteria to use up Sulphur and produce toxic
compounds like smelly H2S (flow needs to be increased). These
denitrator systems (if working properly will) are great to reduce
nitrates in large systems, but as you see, the RedOx potential
should be monitored manually or automatically to exclude toxic
water in the tank.> PS, looking forward to bending
Anthony's ear off in Durban in a couple of weeks. <I'm
sure he is looking forward to that, too. Cheers, Marco.>
|
|

|
Sulfur De-Nitrator A make shift version, will it work -
03/03/09 I have been scouring thru your forums trying to find
more information about these de-Nitrator. As of yet, it doesn't
seem that anyone there has any more info than the basic explanation as
to what it does. I have a 5 year old 75gal reef with a 40 gal sump
<http://www.talkingreef.com/forums/diy-projects/8213-sulfur-denitrator-build.html#>/refugium.
(most soft corals and about 8 med sized fish) and I am board
<Heeee!> with so many water changes. I wanted to get into the
Sulfur De-Nitrator for the ability to not have to change water so much
and also to not be so darn particular when feeding my fish/corals
(worry about waste/nitrates) I had also read that the ARM reaction to
the acidic output will supply some calcium to lessen the frequency that
I dose Kalk. <This is so> I purchased 2 ViaAqua Poly Reactors:
ViaAqua Poly-Reactor (Multi-Media
Reactor)<http://www.marinedepot.com/ps_ViewItem.aspx?category=ViaAqua_Poly_Reactor_(Multi_Media_Reactor)_Saltwater_Aquarium_Supplies_
Filters_Inline___Specialty_Filters_Phosphate_Reactors&vendor=ViaAqua&SearchStr=va3311&action=
view&idProduct=VA3311&idCategory=FIFRISPR&child=VA3311>
and: CaribSea A.R.M.
Aragonite<http://www.talkingreef.com/forums/diy-projects/8213-sulfur-denitrator-build.html#>
Reactor Media CaribSea L.S.M. Live Sulfur Media 1 Gallon I wanted to
know if this setup will work, I was going to start with a VERY small
amount of Sulfur (1/3 filled) in the first chamber and 2/3rds filled
ARM in the second chamber. I was going to start with a 1 drip/hr rate
<Mmm, what "drip" units? Likely adequately slow... a few
gallons per hour will be fine> and adjust as necessary. (I run a
24hr
PH<http://www.talkingreef.com/forums/diy-projects/8213-sulfur-denitrator-build.html#>
monitor) I wanted to also know if anyone has heard any long term
effects with running this setup, like Sulfur buildup in the main tank
after months/years? <Mmm, not likely, no. Sulfur reactors have
actually been "around" for several years (mainly in W.
European use)... they are a tried and true technology> Since most
pumps will have too much power for the 1 drip/sec requirement, <Oh,
here it is> I also had thought about putting 6 foot of tubing from
the tank to the 1st chamber to let oxygen dissipate before the sulfur
chamber and a 6 foot tubing after the second chamber to the tank to
make sure sulfur does not reach the main tank, is this over thinking
it? <Mmm, I think so... but try this out and see> Also, do I need
to create some sort of gas release value for any nitrogen output in the
process? <No... this bit of inert gas will/would just be driven out,
released to the atmosphere> Thank you for any help/suggestions, I
will send pictures after the setup is complete. Gary <Please do
Gary... along with snapshot/s of your water quality test data over
time. Bob Fenner>
Denitrators And Nitrite 8/27/09
Bob,
<James with you today.>
I am in the process of setting up a coil/sulfur denitrator and am
having a slight problem. Even at a slow drip of 1 drop per 3 seconds, I
get almost 1ppm of nitrite. Is it safe to assume that the anaerobic
colony is insufficient and only stripping off one of the two oxygen
molecules from the nitrate? If so, would it be okay to allow the system
to go to a complete stop allowing the reaction to go completely to the
right. I know hydrogen sulfide may form, but the bacteria colony would
increase, wouldn't it?
<No, once you smell hydrogen sulphide, anaerobic conditions have
been reached and the reactor will not work properly as the denitrator
operates under anoxic conditions.>
Once I smell the rotten eggs, I could start introducing fresh nitrates
and expose the effluent to my Sander Ozonizer to oxidize the hydrogen
sulfide.
Another alternative would be to run the denitrator on an empty QT and
feed the colony some skimmate. Any golden nuggets of wisdom you could
throw my way would be greatly appreciated.
<It will take some time for anoxic conditions to be established
within the denitrator so do be patient. It is possible to speed up this
process by the addition of a carbon source to the water. This carbon
source will be processed by bacteria with3in the unit, using oxygen up
and generating the low oxygen levels needed for proper operation. This
can be done by adding about 25 milliliters of vodka (please do not use
Absolut Vodka, is a waste of good vodka) or a sugar solution to the
denitrator column.
Let the circulation pump run a day or so making sure no new water
enters the column. This process should lower the ORP to a usable level
in that time frame.
Then, start with a very low flow rate and check daily for any hint of
hydrogen sulphide.
If it is detected, the denitrator will require more oxygen and a small
increase in flow rate will be needed. This is the "fine
tuning" period, and may take quite some time to tune in properly.
If, after 4 to 5 weeks of operation, and no nitrate reduction occurs,
you will need to reduce the flow rate a very small amount, and this may
have to be repeated if after 2 to 3 weeks, no nitrate reduction is
noted. This is the negative side of sulphur nitrate reactors, time
consuming to properly set up and is the main reason I have never
incorporated one in my system. Once the sulphur denitrator is tuned and
running, keep an eye on alkalinity and pH. Alkalinity will be used up
faster than without the denitrator in operation, so an increase of
alkalinity buffers may be needed. A RedOx meter that can read negative
values is a very useful tool for fine tuning denitrators. The ideal ORP
range would be between -100 to -250 with -170 being optimum. Above -50
indicates too much oxygen for the denitrator to function properly, and
below -300 indicates the water has reached anaerobic conditions, at
which point hydrogen sulphide will be produced.
Perhaps other crew members may chime in here with their thoughts/ideas
on setting up/fine tuning sulphur denitrators.>
Regards,
<You're welcome. James (Salty Dog)>
James Miller
Okayama, Japan
PS. I am going to Kochi, Japan tomorrow to hunt for a small round belly
cowfish and some Zoanthids. :-)
Re Denitrators And Nitrite 9/9/09
James,
<Hello James, like that name.>
I am finally getting zero nitrites and nitrates out of the
denitrator.
<Great.>
It appears my combining a rather long coil (7 meters of 6mm ID airline)
was overly effective at removing the O2. This plus the double reaction
chambers meant almost 10 meters of travel, so it went anaerobic.
Speeding up the effluent with a small colony meant nitrite. I sped up
the drip and allowed nitrite to escape into an ozone reaction chamber
returning any nitrite back to nitrate. The increased flow appears to
have allowed the colony to multiply as I can now process a gallon an
hour with zero nitrates or nitrites.
I guess patience was the key.
<Very much so with these type systems.>
Hopefully I can now keep my nitrates low enough to keep my corals
happy. I thank you for your advice.
<You're welcome, and thank you for the follow up.
James (Salty Dog)>
Regards,
James Miller
Okayama, Japan
Sulphur with a carbon source... Tricky Q's re
denitrators - 10/06/2009
Hi my name is Steen... i have more then one question, but they are
related, so here goes...
I read an article that you can start up the bacterial culture in a
sulphur reactor much quicker by dosing vodka, and just letting it
recirculate for 3 days (can't find the article again, REALLY
annoying!). I can't really find the information i want anywhere,
and i have posted a similar thread on a LOT of forums, no useful
replies! I want to dose a carbon source and run a sulphur reactor at
the same time:
1) Would it be beneficial to dose it slowly through the sulphur
reactor?(diluted form dripping slowly into the reactor intake)
<IF carbon is limited...>
2) Would it work kinda like a combined carbon based reactor with a
sulphur based?(i don't know enough about the differences in the
cultures living in carbon based and sulphur based reactors)
<Mmm, not if I understand your question... No... the ethanol will
only further chemically feed the sulphur-based bacteria>
3) What possible Long term effects could there be when combining
them?(the sulphur beads getting covered with a culture living of the
carbon and thereby not utilising the sulphur)
<Mmm...Combining the C2H5OH? When something becomes rate-limiting...
reaction series will slow>
4) Is the RedOx the same in both types of filters?
<Close>
5) What difference would there be between using VSV and vodka?
<Price?>
5.5) My skimmer is acting strange after i started, most of the time it
does absolutely nothing, and some times it just go crazy, not like more
'dry' foam being created, just the water/foam level rising and
overflowing the cup in no time!?!?
<To be expected>
Hope you can help me out with some information on the subject(s), and
if not then thx 4 your time anyway :)
p.s. I actually started 3 days ago to dose 30ml vodka a day very slowly
into my 18kg sulphur reactor(cleaned 3 weeks ago, so not really matured
yet, trying to keep it around -170mV) for my 900l tank which also runs
a reactor with 1000ml Rowaphos and a reactor with carbon and shuran150
skimmer...
<I'd reduce this to 10 ml.s maximum. Bob Fenner>
Re: sulphur with a carbon source
10/7/09
i Bob, first of all thanks for your answers :) So what i get from it is
that if carbon isn't a limiting factor there'd be no advantage
in dosing it trough the sulphur denitrator,
<Yes>
but you would limit it to 10 ml 37% vodka pr. day in a filter
containing 18kg sulphur beads, why is that?
<IF there is too much Vodka added (beyond the metabolic use of the
microbes in the denitrator) the excess can/will go on and have
"other adventures" in a system... the lesser of which are
(usually green filamentous) algal profusion. 10 ml.s is likely about
the limit of good you can do here. If you had/have diagnostic tool/s
for determining excess hydroxide concentration, I'd actually
measure/monitor... though you will very likely find that the
"use" of ethanol vacillates...>
If i dose it, still keep the ORP/RedOx potential between -100mV and
-200mV.
<Too low... for the main system. Are you stating for the reactor
discharge water?>
Could there be an increased risk of clogging the sulphur denitrator if
dosing into it?
<Yes; slightly>
I hoped that like carbon based denitrators that use bioballs, the
sulphur beads and aragonite would provide the surface area for the
bacteria to grow on when dosing vodka, but that would not be the case.
And using pure vodka has no advantage over VSV other then being more
expensive, since sugar and vinegar are really cheap!(any drawback with
VSV?)
<None that I'm aware>
Have you heard about 'quick restarting' a sulphur denitrator
with a carbon source?
<I have, and discount it/this. In most any established system there
is sufficient carbon in place to initiate denitrification in these
units w/o supplementation. I would make an equivalent statement with
the forward reactions of nitrification and presence of sufficient
nitrogen compounds.>
and again thanks for the advice you've already given, and thanks
for your time..Happy Landings... Steen..
<You too. BobF>
FW: sulphur with a carbon source
10/7/09
Hi the unit is behaving totally weird now, it fluctuates A LOT when
power i disconnected
<!? I would NOT disconnect power here... like "the spice"
(Dune/Herbert), the water must flow>
and connected, so by disconnecting it i can get any value i want, plug
it back in and everything looks all right, except the aquarium ;) ... I
have tested it on a solution with a known RedOx and it indicated
-40mV
<Danger! You do NOT want negative ORP>
in a 300mV solution, problem is that i costs me 40 dollars to receive
the package every time, because it is from outside the EU and 15 to
send, so if i send it back and you repair the unit at you send it back
to me, it will cost me 95 dollars in total excluding the price of the
unit, don't really know what to do here, hope you can help me
somehow...
<? Am not following you... What package? Media/stock feed?
BobF>
FW: sulphur with a carbon source
sorry mate, was not intended for you, my RedOx unit is broken :)
<What a relief! Of life, living things in our world, we don't
want negative ORP. Cheers, BobF>
|
|

