|
FAQs about Health/Diseases, Pests of Soft
Corals of the Family Alcyoniidae 14
FAQs on Alcyoniid Disease:
Alcyoniid Health 1,
Alcyoniid Disease
2,
Alcyoniid Disease 3,
Alcyoniid Disease
4, Alcyoniid
Disease 5, Alcyoniid
Disease 6, Alcyoniid
Disease 7, Alcyoniid
Disease 8, Alcyoniid
Disease 9, Alcyoniid Health 10,
Alcyoniid Disease
11, Alcyoniid
Health 12, Alcyoniid Disease 13,
Alcyoniid
Disease 15, Alcyoniid Disease ,
FAQs on
Alcyoniid Disease by Category:
Diagnosis,
Environment,
Nutritional, Pathogenic (Infectious,
parasitic), Predator/Pests,
Social, Trauma,
Treatments
Related Articles: Soft Corals of the Family
Alcyoniidae
Related FAQs: Soft Corals of the Family Alcyoniidae,
Alcyoniids 2, Alcyoniids 3, Alcyoniids 4, Alcyoniid ID, Alcyoniid Selection, Alcyoniid Compatibility, Alcyoniid Systems, Alcyoniid Behavior, Alcyoniid Feeding, Alcyoniid Propagation, Soft Coral Propagation, Soft Coral Health, Dyed Corals, Soft Coral Propagation, Nephtheids, Dendronephthya, Paralcyoniids, Nidaliids, Xeniids, Dyed
Corals,
|

|
Devils hand. 7/10/18
Good afternoon Bob,
<Hiya John>
I was wondering if you could weigh in on something. I have a HUGE devils
hand in my 750 that I’ve had for about 6 years. He’s about 23” across
and 14” tall. He’s always been healthy and no issues that I know of.
<Good>
Over the last couple weeks I’ve noticed that one of his lobes is turning
a blackish color and there are 2-3 other spots in different areas doing
the same thing but the other 2-3 spots are about 2.5-3cm in diameter. He
is still fully open and great polyp extension except in those areas. Any
thoughts on what’s going on?
<Mmm; may be nothing... but... if these areas turn out to be obviously
necrotic (wearing through, expanding), I'd consider excising (cutting
them out); IF the whole colony suddenly appears to be collapsing, I'd
frag several areas of good tissue and toss the rest; raise the frags in
a few systems>
I’ve never seen this before.
<Unfortunately, I have>
Thank you
John
<Welcome. Bob Fenner>
Re: Devils hand. 7/10/18
Thanks Bob. If I get a chance tomorrow when the lights are on I’ll get
you some pics of the areas.
<Ah, good. B>
Thank you.
John
<Oh! John, do try "rubbing the spots off" with your finger/nail. BobF>
Re: Devils hand. 7/10/18
Will do. Thanks Bob. If it removes with my fingernail does that mean
algae or a fungus?
<Mmm; that it's likely something (more) superficial; less worrying. Bob
Fenner>
|
PowerPoint on Allelopathy 1/18/17
Good evening,
<TT>
I have a green leather toadstool with patches of white opaque areas on
the "face" that the polyps extend out of. It is opening and extending
its polyps as it would normally and seems in good spirits despite the
white patches. My first guess is that it is trying to "shed" as leathers
will do, but I am more concerned for the safety of the other corals in
the tank. If
you upset the leather it can secrete toxins so I want to keep it as calm
as possible. Will send pictures if you like.
<Please do; well cropped... a few hundred Kbytes>
I remember reading that you had a PowerPoint on allelopathy but I can't
find it for the life of me. Would you mind directing me to the link? If
you have any suggested reading for me I would appreciate it. Thank you
so much for your insight and your wonderful website. I've spent
literally hours getting lost here.
<Don't think we have such a ppt... I put away most all... and have for
twenty years.>
S pozdravem / Best regards,
Taylor Thrasher
<I suspect you're right re the Sarcophyton shedding... which they do
almost continuously, then shed a bunch at times. I encourage you to
triple dose iodide-ate, turn up your circulation, aeration; refresh you
chemical filtrants (Chemi-Pure and PolyFilter are faves here... and keep
your eyes on your other livestock for signs of stress. Bob Fenner>
Re: PowerPoint on Allelopathy
1/21/17
Good afternoon,
<Cheers double T>
Thank you for the prompt response. Here are some photos of my mean ol'
toadstool leather.
<Mmm; Oh, I see; they're linked below>
It's been a busy week so this photo was taken only minutes after lights on.
It's "eyes" are still opening but you can see by the photos that it is
quickly responding to light and polyps are expanding to full glory.
I've seen this coral upset when I had to remove all the Aiptasia anemones
when I rescued it. I've noticed this animal is particularly sensitive to
stimulus of any kind but "upset" takes the form of zero to weak polyp
extension and frequently folded in a weird position.
<You're a keen observer>
In the photo you can see where there is some irritation on the stem which I
believe is caused by some fisherman-like worm near its base. It has a
threadlike (not glossy spider web like) tentacle similar to a spaghetti worm
that it touches the base with. If you think this is harmful or a precursor
to the agitation, any suggestions for removal are much appreciated.
<I definitely would remove this... and look about, see if any have bored
into the Sarcophyton near the base itself... AND cut these out if so>
Google: https://goo.gl/photos/QSyaCa6yNVg1L6Bm8
<Nope>
If that doesn't work,
Photo1:
https://www.dropbox.com/s/3nvu70w318aay1p/Toadstool%20Leather%201.jpg?dl=0
Photo2:
https://www.dropbox.com/s/95b7kwp9kcz6au7/Toadstool%20Leather%202.jpg?dl=0
Photo3:
https://www.dropbox.com/s/o3ripa78adicp3j/Toadstool%20Leather%203.jpg?dl=0
<These do>
I purchased some Chemi-Pure and will be implementing that into my system.
PolyFilter is on the way from Amazon. Thanks again for your help.
S pozdravem / Best regards,
Taylor Thrasher
<I would do all that I mentioned in our first email exchange; particularly
the Iodide-ate administration. Bob Fenner>
|
 |
Common Questions but SO IMPORTANT TO ME PLEASE. Sarco,
carpet anem. mis beh. 4/19/13
I have a 140 gallon reef tank with over 150 gallons of water in the
sump.
All Parameters are great, all my hard corals, anemones, etc
<Should I assume you're aware of allelopathy twixt Cnidarians?>
are open and healthy.....but my 2 Tyrees (aust. green leather toadstools)
won't open.
<... Sarcophyton species... Not Steve per se>
It's been 3 months....They are about 6 inches below the water level with
LED lights above. HELP PLEASE HELP
<...>
I've added Iodine, I've put healthy opening green leather frags right
next to them, etc.....Any genius ideas?
<... they/these may have been compromised ahead of your receiving
them... there may be the celebrated (mentioned above) chemical warfare
going on... Esp. among competing Alcyoniids... >
2nd minor question.....Healthy expensive Carpets have moved behind the
rock
<A good clue>
and are open under the lights behind the rock. I can't reach to try and
move which wouldn't be smart anyway.......Any tricks to get Carpets to
move, i.e. turn off lights on that side of the tank or increase annoying
flow to that area, etc....
<Just patience... if anything, moving other stinging-celled life that's
too near them (w/in a foot)... I would be reading re the measure of
RedOx, using ozone likely, chemical filtrants, adding a much, MUCH
larger RDP refugium, humongous DSB to this system... for the reasons
that will become obvious to you while reading on WWM>
Your loyal follower begs for your opinion....
Thanks,
Adam
<Welcome. Bob Fenner>
|
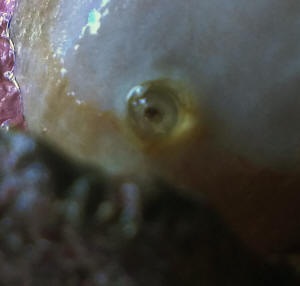
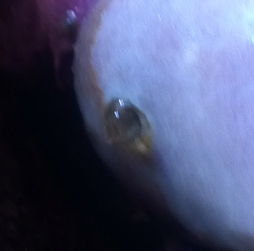
Sinularia with weird "breathing hole"
11/16/11
Hi,
I've a problem with my Sinularia sp...it doesn't look
poorly but it's shedding more often than normal.
At a close look I found a weird hard "cannula"
protruding from stalk, it seems a "breathing hole" of
some endoparasite.
<Mmm, new to me>
Pics will be more eloquent.
What kind of parasite is it?
<I would risk (not big) cutting this out... some single edge
razor blade cuts from the opening edge out>
Thanks,
Manuel Ricci
<Do please follow up w/ your further observations. Bob
Fenner>
|
|
|
Lemnalia sp. doing poorly
11/12/11
Good Morning Crew & thanks so much for sharing your expertise &
experience with others.
<A pleasure, honor to share>
I few weeks ago I changed my filtration system on my 90 gallon reef
from a Pro Clear wet/dry with bio balls to a 30 gallon sump with
refugium. After re-starting the pump there was a ton of bubbles in the
tank. I added a filter sock on the drain which took care of the
bubbles. I began to notice my Lemnalia sp., normally about 5 inches
tall, was very contracted. The "branches" will not extend
either during the day or night. The specimen was a light tan. Now
it's more white. Does this specimen need to be fed anything in
particular to revive it?
<Perhaps... at least the addition/boosting of iodide-ate
supplement>
I noticed that several Discosoma mushrooms had grown around the
Lemnalia, so I moved it to another part of the system where there were
no mushrooms.
The system is loaded with these mushrooms, approximately 100 of
them. I did read on WWM that allelopathy with the mushrooms
can eventually win over the Lemnalia. Is that to say the Lemnalia
can't survive in this tank?
<Is likely losing to the Corallimorphs. I would be
moving this soft coral to another system>
It's now 1 week since I moved the Lemnalia & it shows no signs
of improvement & I've actually seen some black "dots"
on the specimen that I snipped off because they appeared necrotic. I am
running activated carbon in the sump.
I'm wondering if I should try to get rid of it, or if there is
something else I should do.
System parameters are:
90g display with 30g sump
CA= 400
Alk=2.5 meq/l
Sg= 1.023
Am= 0
NO4= 1.0
Temp= 80-82 F
Ph= 8.0 to 8.2 (am to pm)
Phosphate test is empty & on order so I can't be sure.
<Need some; measurable... which you very likely have... given the
health of
the 'Shrooms>
Equipment:
Pump= Rio 3200 (approx 1900 gph)
3 power heads
The Lemnalia sp. gets moderately high flow, but not directly
Lights= 500 w PC
Turboflotor Blue 1000 skimmer
UV sterilizer 25w
GFO reactor
<Mmm, unless there's some compelling reason for using this,
I'd get rid of it>
Thanks for your help folks
Doug S. St Joseph, MI
<Welcome. Bob Fenner>
Re: Branching Montiporas retracting polyps, HPO4 angle
11/12/11
Thanks Bob, I was reading on your site that removing too much phosphate
can also cause these symptoms of starving the corals, true?
<Mmm, can, as well as too little; but you don't have excessive
HPO4, and you report/ed that your other Cnidarians were fine>
I tested my PO4 yesterday with my Hanna checker and it was zero, so I
took my gfo reactor and carbon reactor off line to see if letting the
PO4 build up some to around 0.04 on my checker would help?
<As well as just simple food additions for your other
livestock>
The reason I am leaning that was is based on what my last PO4 reading
was before the symptom started showing up (my recorded PO4 level was
about 0.05). Could the water be too "clean" for the branching
Montis?
<Not likely; no>
Based on the limited information you have about my system, would you
still lean toward the Zoanthids?
<Some form/source of allelopathy, yes. The Zoanthids/Mats are the
most likely suspect of what you list/ed>
The branching type Monti's that are affected are in different areas
of the tank and have been in the same spot for several months before
this occurred.
<As I surmised>
One final thought (change that I made just before this happened) I had
several smaller power heads for flow in my tank, that were not on any
sort of timers or wave makers, so I replaced them and reconfigured my
flow with the two Koralia magnum 7s on an RKE wave maker. This has
provided a LOT more varied flow for my tank and just after making that
change I noticed the branching Monti's retracting all of their
polyps.
<Could be a factor as well>
Just want to mention that none of the Montis are directly in front of
the power heads and are not getting blasted with flow. Could it be the
increased flow is "stirring up" the Zoas released chemicals
causing this?
<Yes>
Based on this limited info about my situation, what would you do in
this
situation?
<Nothing overt. I might increase/pulse whatever source of iodide-ate
you utilize, as well as the items mentioned in last email...>
Looking forward to your responses.
Thanks,
<Welcome. BobF>
|
Leather Tree's Melting... Too little info.
9/15/11
Hey Guys,
<Jason>
I came home to find my two leather trees melting away in my tank.
I have had these for two years and have been great up until say 3
days ago. They shrunk down and I thought they were doing their
normal shrink down then expand back out thing but tonight they
are falling apart. I snapped these pictures, I have more than
this but figured these would be a good start. I checked all of
the parameters and they are all within normal limits
<... need numbers>
and have been the same for the last several months. The trees
have been in the same spot for months, nothing new has been added
to the tank in 6+ weeks,
<... and to know what other stock is here...>
haven't changed
anything from our normal routine lately. So any ideas how to
salvage these?
<... Not w/o data>
If you need more pictures or closer shots please let me know.
Thanks,
Jason
<Mmm, peruse here: http://wetwebmedia.com/AlcyDisF14.htm
and the linked files above in the series. Likely the issue here
is either chemical or environmental... Bob Fenner>
|
[1]%20leather%20melt.jpg) [1]%20lethr.jpg)
Re: Leather Tree's Melting
9/16/11
Ammonia 0
Nitrite 0
Nitrates <5 down from 20 about 3 weeks ago.
Salinity is 1.026. Phosphates .04
Stock is as follows
3 Chromis
Yellow Tang
Hippo Tang
Kole Tang
2 clowns
2 Mandarin
2 blennies
2 Dwarf Angles
There are a assortment of corals
1 closed Brain
Several Ricordea
Several colonies of Zoas
Several leathers but only two effected
Assortment of sps
Frogspawn
Blastos
Hope this is the info you were looking for
<Mmm, well, am leaning more toward a cascade even/allelopathy.
Do read where you've been referred. BobF>
|
|
Toadstool Help 8/24/11
Dear Crew,
<Justin>
I bought a nice toadstool from my LFS thinking it would be a
great addition to my 125g. I drip acclimated it over night, did
my normal Lugol's dip,
<Mmm, maybe burned hence...>
and it looked perfectly fine, polyps extended and everything. I
placed it about 8" under my lights (T5 4x39w) and continued
about my business. A few hours later this is what he looked like:
DSC_8424.JPG
<Beat up... chewed, or...>
I promptly placed him at the bottom of the tank, and am awaiting
to see what happens. I have a neon green toadstool, and it's
doing great, so I am not sure what is going on here. Please let
me know what more I can do. Thanks!
Sincerely,
Justin
<Likely allelopathy at least at part at work here. Use the
search tool on WWM with the string: toadstool coral
allelopathy and read. Bob Fenner>
|
|

Re: Toadstool Help 8/24/11
Dear Mr. Fenner,
<Justin>
Thanks for the plethora of information. Though the toady never
came in contact with anything,
<Don't have to touch... Alcyoniids and others produce
copious chemical warfare material>
I can think of, I am wondering if the 100+ polyps of
Lavender Hairy Mushrooms might have been the culprits.
<Ah yes>
The original spot was at least 6 inches away from anything, but
you never can be quite sure. To be safe, I hooked up an old
Marineland Magnum 350 as a way to do some carbon filtering, in
the event there is something that is in the water column it
doesn't like (from the mushrooms, or perhaps the mini carpets
I have). I have included a picture, as the toady has seemed to
respond well to the new placement, and addition of carbon
filtering. Thanks again! I appreciate all the help. Hopefully in
the coming weeks I will see it turn around.
Sincerely,
Justin
<Mmm, well, I'd be moving this animal to quarantine,
mixing water as gone over in my review/ppt on Cnidarian
allelopathy:
http://wetwebmedia.com/CorlCompArt.htm
and the linked files above. BobF>
|
Cabbage Leather...At a Loss For
What To Do/Sinularia Health 1/11/11
Hello WWM,
<Hello Wes>
I love reading and finding out all the cool info on your site!
<Glad you enjoy and thank you.>
I have an unhappy and, I fear, dying Cabbage Leather Soft coral. I got
this coral 11 months ago along with a Finger Leather coral. The Finger
leather is on one side of the 220 tank and The Cabbage the other. The
Finger Leather has grown from 6 inch across to over 10 inches in 3
different ways. The cabbage leather went from a 1.5 inch circular frag
to over 7inches.
<Nothing wrong with that.>
About 6 weeks ago both leathers began to go downhill. My tank
parameters/maintenance had become laxed over the busy Autumn.
<Can lead to problems.>
The Phosphates had increased to 0.05+ and a brown algea <algae>
was growing.
The algea <algae> is slimy and will grow up to 6 or more inches a
day in long strings, sometimes. It is not like any of the cyno
<Cyano> that I had ever had or seen. My pH was 7.8, the Alk was
below 7 dKH.
Since then, I have done some major cleaning to the RO/DI unit, UV
sterilizer, and swapping out some of the overgrown live rock with some
clean rock from in the sump. The system is a 400 gallon multi tank set
up, with Pre-Sump+Skimmer, Sump+Remote DSB & live rock chambers,
Refugium, 2 small display tanks with no algae problems and the main 220
display.
The stock in the 220 is:
Many Mushrooms (growing and multiplying like crazy the past 4
months)
Finger Leather
Cabbage Leather
4 Kenya Trees (plus a dozen+young all over)
Yellow Tang
Azul Damsel
Sunset Pseudochromis
Spotted Mandarin
Royal Gramma
Cinnamon Clown
Scooter Dragonette
All fish are 11 months to several years old.
2 Bubble Tip Anemones (was one until a few months back when it
split)
<No overstocking for sure, one good thing.>
a growing number of glass anemones (I bought some Aiptasia-X
<Aiptasia> to try to check these)
I have brought the tank parameters to this:
1.026 specific gravity
78 F
8.0 PH
0 ammonia
0 nitrites
2 nitrates
0.025 phosphates
11.2 dKH
260 calcium
1420 magnesium
Everything is looking good now except for the cabbage leather. It is in
constant 'shedding' mode. The Finger looked bad for 2 weeks
last month but now it is fine. I am thinking about moving the Cabbage
leather to my refugium.
<What is your total flow rate in the 220?>
I cannot get my pH up with Buffer, dosing Kalk (one time) and 2 part
Alk/calcium additives. My calcium has never been that low. (normally
350)
Have I messed up the ionic balance? I made sure not to add Calcium
chloride.
<Likely, you were just lax on dosing as well as tank maintenance.
Nothing wrong with using Calcium Chloride as a quick boost, but would
not use it continually.>
This gooey, stringy brown algae is still present, but I am beating it
back with all the water changes and siphoning. I only need to siphon it
off once a week to keep it under control, whereas early on I needed to
do it multiple times.
<This will take time to eradicate, didn't happen overnight and
will not cease overnight.>
The Cabbage leather never had any problems till this algae
showed...could it somehow interfered with its own algae?
<Unlikely, more to do with water quality going downhill.>
I read online that they have a symbiotic algae. I also read that they
need iodine. I wonder if I should get yet another additive.
<What type lighting are you using? In most cases, Sinularia, which
is a very hardy coral, fair better in brighter light and moderate to
strong water flow which help rid them of
any tissue being shed. You should get enough iodine with regular water
changes to satisfy any requirement for this element.>
Any ideas or thoughts would be appreciated....as I don't want it to
perish.
The Refugium is not the best place for it...but it is free of that
brown algae.
<I would continue good maintenance practices and give it some time
to recover.>
Thanks
<You're welcome. James (Salty Dog)>
Wes
|
Degrading Corals 1/3/11
Dear WWM crew,
<... Nick... we ask people to limit image file size... to a
few hundred Kbytes... you, 10 megs... 20% of our mail
capacity...>
Happy New Year! I have a 90 gallon reef system that has been
running for nearly a year now with no problems. Last week my
water began to get more cloudy than usual, so I did a 15% water
change (RO water, of course), which cleared up my problem. Since
completing the water change however, I have a much larger crisis;
some of my corals are looking pretty bad!
<I see this>
I have had (what I believe to be) a colt coral (picture
attached), a (confirmed) flower pot coral, and two Ricordea for
almost eight months with no problems; they all have been healthy
and thriving. In fact, the 'colt' coral has more than
doubled in size in that time and my two Ocellaris clowns have
been hosting the Goniopora since its introduction. Since the
water change, the flower pot only partially opens
('blooms') my colt coral - once perky and spread out - is
drooped over and clumped together, and the Ricordea is about 1/3
its typical size. When I introduced the new water during the
water change, I made sure to pre-mix the salt and PH buffer in a
bucket to be sure it matched what is in the tank and ran a pump
for a few
minutes to mix everything together.
<Fair to good, but much better to pre-mix, let sit,
recirculate for a few days ahead of use>
My SG is 1.024, temp is 79-81, calcium is 490ppm,
<Really too high... and in relation to Mg, alkalinity?>
oxygen is fine (I forget the exact numbers), nitrates are 0,
<... an essential nutrient. Your corals need some>
nitrites are 0, phosphates are 0,
<And this>
ammonia is 0. I know the problem is not salt burn because the
water was put into the tank with a hose (stayed in one spot) and
the affected corals are spread over different sections of the
tank. My lighting is a 6 -- bulb H.O T-5 setup with 3 actinic and
3 white; 354 watts total. My system also contains an open brain
coral, a Derasa clam (2.5 -- 3'), two purple flat blade
gorgonians, a host of green mushrooms, Zoanthus, an anemone --
unknown species - (brown with pink tips; about 3' in
diameter), a green bubble
tip, a flame scallop,
<Hard to keep... Along with the Goniopora; you must be doing
much right>
a feather duster, a Strawberry Conch, a Red Sea Star, and a few
fish; all thriving and all present during the water change.
I'm worried I am going to lose the corals in question and any
advice would be much appreciated!
Thank you,
~ Nick
<Likely a combo. of disproportionate Ca conc. w/ Mg, Alk...
and def. a starvation issue with a lack of NO3 and HPO4... Could
be quite a few "other things"... You would likely do
well to read here: http://wetwebmedia.com/CnidDisF3.htm
and the linked files above for background, as well as
investigating (the search tool, indices on WWM) the central
issues mentioned. Bob Fenner>
|
|
 
|
|
Colt coral frag health. 12/13/10
Good morning!
Hope this email finds you well.
Thanks for taking the time to answer my questions.
I have a 75 gallon with a 20 gallon sump. Lighting is 2 10000k
ice white t5s and 2 true blue actinics. My parameters are ammonia
0, nitrite 0 and nitrate 0.
<Nitrate is an essential nutrient for many
chemoautotrophs...>
My ph is constantly 8.0 and I am in the process of raising it to
8.3 with the use of Kent pro buffer dKH.
<Not important here; and do take care w/ how you're
adjusting>
I will be purchasing an alkalinity, calcium and phosphate testers
in the near future.
<Good>
I received a frag from a friend two days ago of a colt coral. I
have attached him to the rock via an elastic band. He has
expanded, during the days, since I've received him but no
polyp extension at all. He appears to be bleaching on some of the
tips of his branches.
<I see this>
I have not found a whole lot of information about colts bleaching
when skimming through the FAQs.
I know it has only been a couple days. The aquarium has been up
and running for about three months. I do 5% water changes twice a
week. I just started to run active carbon, from Marineland as
well.
Thanks,
<Likely, in addition to general stress from being moved, a
lack of nutrient. What are you feeding this organism, system? Do
you have a "dirtier" longer-established system to move
it to? Bob Fenner>
|
|

|
|
Re: Colt coral frag health. 12/13/10
I feed the system roggers reef food and new life spectrum
pellets.
<Good foods I'd merit>
I also received a Kenya tree coral. It's doing really good.
All the polyps are extended during the day. I have two percula
clown fish and a flame angel.
<Ahh, a good clue>
I do not have another system to put the colt into.
Purchased the phosphate and dKH testers today. Will test when I
get home.
Thanks for getting back to me!
<I urge patience here. Chances are this Sinularia will rally.
BobF>
Re: Colt coral frag health. 12/13/10
As far as raising the ph. I just follow the manufacturer's
directions.
Is this a good product? Would you recommend another one? Or
another way? I already opened the windows but the ph did not
change.
Thanks again for your help!
<Read here: http://www.wetwebmedia.com/marine/maintenance/maintindex.htm
scroll down to pH, Alkalinity...
B>
Re: Colt coral frag health. 12/13/10
After reading the FAQs you've recommended, on more than one
occasion, that a consistent ph o 8.0 is fine.
<Yes>
I will back off as I can see over the three days of use that one
of my fish is starting to breath more rapidly.
<Only add such supplements to new/make-up seawater (not
directly to established systems) and allow to set/circulate a
good day ahead of us>
I will stop adding and see if this was the cause.
Thanks
<Thank you, BobF>
|
|
Green Finger Leather
Bruised?/Alcyoniidae Health 11/14/10
<Hello Astrid>
I know that you get tons of questions so I will try to keep this
brief. I have a 20 gallon Nano tank. The pH is a bit low 8.1 and
the alkalinity is low also 2.7 me/li <meq/liter and is not low
(7.5 dKH).>. I am using expired tests and have new ones on
order. The not expired, dip stick tests show pH between 8.0 and
8.4 and the alkalinity between 240 and 300 ppm.
<Ballpark figures with dip strips.>
I have some buffer but don't want to use it until I get test
that I have better confidence in. All other parameters are good
and stocking is low.
If you need more info on my system, please let me know.
I have a Green Finger Leather Coral that was not attached to any
rock. I tried strapping it down with cheese cloth. It looked
miserable. I tried balancing it in a rock. It kept falling off. I
did some research on your site and decided to sew it to the rock.
Last night, I did the sewing in a bucket with clean, aerated
saltwater and added a heavy dose of iodide. I used a small needle
and fishing line. I put three stitches less than an inch from the
bottom and around the rock. The crown looks okay and it is
staying in place well, but the base looks bad.
<This will take some time before the coral attaches, will not
be overnight so to speak.>
The first picture if from the day before the sewing. The base is
whitish, plump and firm with coarse "hairs" at the
bottom. The second picture is what it looks like now. The whitish
area is higher up than it was, it has shriveled up and has dark
brown spots. Is this a problem or is it just bruised? Is there
anything I need to do?
<I see no pictures, please send, then I will respond. James
(Salty Dog)>
Thank you,
<You're welcome. James (Salty Dog)>
-Astrid
Re Green Finger Leather Bruised?/Alcyoniidae Health
11/14/10 - 11/15/10
Hmm, I'll try that again.
<Mmm, take a look at the photos you sent, heavy pixelization,
almost as though the photos were censored. James (Salty
Dog)>
Green Finger Leather Bruised?/Alcyoniidae Health
> <Hello Astrid>
> I know that you get tons of questions so I will try to keep
this brief.
> I have a 20 gallon Nano tank. The pH is a bit low 8.1 and
the alkalinity
> is low also 2.7 me/li <meq/liter and is not low (7.5
dKH).>. I am using
> expired tests and have new ones on order. The not expired,
dip stick tests
> show pH between 8.0 and 8.4 and the alkalinity between 240
and 300 ppm.
> <Ballpark figures with dip strips.>
> I have some buffer but don't want to use it until I get
test that I have
> better confidence in. All other parameters are good and
stocking is low.
> If you need more info on my system, please let me know.
> I have a Green Finger Leather Coral that was not attached to
any rock. I
> tried strapping it down with cheese cloth. It looked
miserable. I tried
> balancing it in a rock. It kept falling off. I did some
research on your
> site and decided to sew it to the rock. Last night, I did
the sewing in a
> bucket with clean, aerated saltwater and added a heavy dose
of iodide. I
> used a small needle and fishing line. I put three stitches
less than an
> inch from the bottom and around the rock. The crown looks
okay and it is
> staying in place well, but the base looks bad.
> <This will take some time before the coral attaches, will
not be
overnight
> so to speak.>
> The first picture if from the day before the sewing. The
base is whitish,
> plump and firm with coarse "hairs" at the bottom.
The second picture is
> what it looks like now. The whitish area is higher up than
it was, it has
> shriveled up and has dark brown spots. Is this a problem or
is it just
> bruised? Is there anything I need to do?
> <I see no pictures, please send, then I will respond.
James (Salty Dog)>
> Thank you,
> <You're welcome. James (Salty Dog)>
> -Astrid
Re Green Finger Leather Bruised?/Alcyoniidae Health
11/14/10 - 11/15/10
The pixelization was probably due to size reduction. Here they
are in full size. It still looks the same.
<Doesn't look the same on this end, looks good here. I
believe your coral just needs some time to attach, would not
worry too much at this stage. I might add that the "heavy
dose of iodide" likely didn't help matters. Overdosing
this element can be lethal.>
Thank you.
<You're welcome. James (Salty Dog)>
|
|
  Dyed Dyed
|
|
Toadstool leather questions 11/5/10
Hi!
<Lea>
I've had a toadstool leather (Sarcophyton sp.) for about 3 or
4 months now and he's been doing well. Recently he did the
whole waxy shedding thing that I learned about on your FAQ. He
seemed to rebound quickly after that, especially after I used a
pipette to blow off the waxy bits and speed up the process. He
slouched a little during the process, but I didn't think
about it as I figured it was normal. Its been about 2 weeks since
the shedding and he seems to be slouching again, but the polyps
are still extended. He's
grown a little bit since I got him, with the crown circumference
increasing by about ½ however, I
fail to note any appreciable increase in the stalk
size. Could the slouching be due to the crown weighing down the
stalk?
<Not really>
I also noticed a patch of brownish tissue near the back of the
stalk at the base (on the opposite side from the direction of the
slouch).
<I see this... the other life about the base of the pedicle
concerns me... it may be chemically competing w/ the
Sarcophyton>
Its not black, but a mauve-ish color (a few shades darker/browner
than the normal pastel pink. Could this still be tissue necrosis
(picture attached)?
Should I cut off the stock? If so, how do I attach it to a piece
of rock?
<I would remove the other (Corallimorph?) life from/near the
base instead... but notes on cutting/fragging Alcyoniids can be
easily found on WWM>
Another option is that, due to the growth, he could be getting
too close to the surface and lights. I have a JBJ 24g deluxe
aquarium and because of the PC lighting I placed him very near
the top. I've always noticed that the polyps at the top
don't open up all the way (they extend, but you don't see
the �flower�), while the polyps
at the bottom do. Could he be bending down/slouching to get away
from the light?
<Again, not likely>
I'm reluctant to move or cut in case I should just let it
be.
Thank you!
Lea
<Welcome. Bob Fenner>
|
|

|
|
Re: Toadstool leather questions
11/8/10
Hi Bob,
<Lea>
Thanks for the advice. The Ricordea florida are actually not too
near the base (at least 6" away), but the perspective makes
them look closer. I know 6" isn't very much, but in a
smaller system it's hard to get much farther away (though I
was able to move them a few more apart). I do run carbon all the
time and change the bag monthly. The brown patch is actually on
the opposite side of the R. florida, though I guess it could just
be a sign of a pathogen and the allelopathy is making it worse. I
will watch it over the next couple of days and make a decision
if/when I see a change.
<Ok>
I've seen various FAQ on how to frag, and if I have to am
planning on cutting above the bad tissue, doing an iodine dip for
7min (100mg iodine per gallon), and then reattaching. My question
is actually about how specifically to reattach.
<I like thread, thin monofilament, but know folks who spear w/
toothpicks (plastic), use cyanoacrylates, just drop cuttings on
suitable rubble landscape to attach on their own...>
I've looked up a few coral fragging videos and they all seem
to deal specifically with the type of fragging that concerns
pieces of the head of the coral. I'm hoping to preserve at
least some of the stalk and use that as the "suggested"
site of attachment. Do you have any recommendations on how best
to attach the stalk to a smallish rock (so I can glue it in place
after recovery)?
<"Tie your mother down...">
I'm not sure how a rubberband or tying it will work if
I'm trying to tie down a vertical structure rather than over
a flat/horizontal piece. I've seen one video that pierces the
frag with a plastic toothpick and then uses a rubberband to
fasten either end of the toothpick down (going behind the rock),
but I'm afraid of sticking the tissue with a toothpick and
creating more problems. Is this a common enough practice to
trust?
<Mmm, yes>
Thank you!
Lea
<Welcome. BobF>
|
|
|

